Which Best Describes This Reaction Between Magnesium and Oxygen
2 Mg O2 2 MgO. Sodium will also react with oxygen.
The Change In Mass When Magnesium Burns Experiment Rsc Education
For the reaction between magnesium and oxygen describe the reactant appearance and identify the evidence of chemical reaction.

. M gO2 M gO M g O 2 M g O. This reaction is extremely exothermic releasing a great deal of heat and light which is why magnesium fuses are used to initiate reactions such as the thermite reaction and. Per second 20 5 40 0125.
The ionic bond between magnesium and oxygen is stronger than the ionic bond between. An oxygen atom will gain 2 electrons to form a stable 2-ion. _ Mgs - 020 - Mgs Type of.
Magnesium is a group II metal and therefore has two electrons in its highest energy level or outermost electron shell. However unlike magnesium sodium reacts immediately when it is exposed to oxygen and produces very little heat and no visible light. Rust is a form of iron oxide and it forms slowly when iron is exposed to air.
When magnesium reacts with oxygen it produces light bright enough to blind you temporarily. July are based on the reaction between magnesium and oxygen to form magnesium oxide. As a result of this exothermic reaction magnesium gives two electrons to oxygen forming powdery magnesium oxide MgO.
Fire is what we call the heat and light produced when things burn. The reactions with oxygen. Which of these would BEST describe the structural difference between magnesium and aluminum.
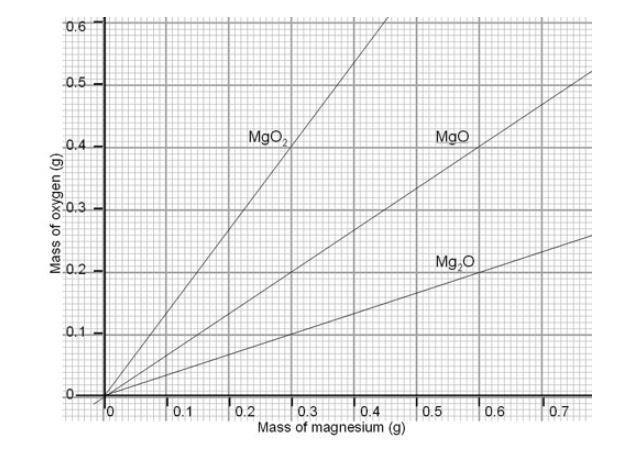
For the reaction between magnesium and oxygen describe the reactant appearance and identify the evidence of chemical reaction. Draw a line of best fit on the graph. Magnesium reacts with oxygen to form magnesium oxide.
AMagnesium has one more proton than aluminum. 2 Mgs O2g 2 MgOs The balanced equation for this reaction can be used to construct two unit factorsthat describe the relationship between the amount of. A magnesium atom will lose 2 electrons to form a stable 2 ion.
In the following questions you will be asked to provide some details of the chemical processes involved in a reaction between magnesium. IiSuggest two reasons why all of the points do not lie on the line of best fit. The term oxidation was originally used to describe reactions in which an element combines with oxygen.
2Mg O2 2MgO It is an oxidation reacti view the full answer Previous question Next question Transcribed Image Text. BI ITU BEIT IN fx1 ble Reactant Reactant appearance Evidence of chemical reaction Mgs Balance. When magnesium burns it combines with oxygen O2 from the air to form magnesium oxide MgO according to the following equation.
Some students burned magnesium in excess oxygen as described by the equation 2Mgs O2g triangle thingy 2MgOs They recorded their data in the table below. In this example the electrons are shown as dots and crosses. Reactions involved the transfer of electrons from one atom to another.
The unbalanced reaction between magnesium and oxygen is. When the reaction with oxygen occurs these two electrons are donated by the magnesium forming positively charged Mg 2 ions. 2 Mg O22 Mg2O2- In the course of this reaction each magnesium atom loses.
The chemical reaction beyween magnesium and oxygen is given below. The process of respiration produces energy for organisms by combining glucose with oxygen from the air. 2Mg CO2 2MgO C Which statement describes what happens in this reaction.
Oxygen is in group 6 of the periodic table. Then balance the chemical equation and identify the type of reaction. The reaction between magnesium and carbon dioxide is shown in the equation.
Attachment Reactant Reactant appearance Evidence of chemical reaction Mg s Balance. What is a balanced chemical equation for magnesium reacting with oxygen to produce magnesium oxide. In Lesson 20 a magnesium strip was used to ignite the thermite reaction.
The term oxidation was originally used to describe reactions in which an element combines with oxygen. You will often see electrons drawn like this in books. 2 Mg O 2 2 MgO.
When magnesium burns it is actually reacting with oxygen in the air and not with fire. The reaction of aluminum metal with oxygen gas typically produces the product with. Combustion reaction takes place between the Mg and O2 proving there is a chemical reaction.
Rust can be prevented by coating iron surfaces with paint or with rust-resistant metals such as chromium or zinc. 1 Mg is whitish-grey in color and MgO formed is white in color. 2Mg s O2 g 2MgO s Magnesium oxide is an ionic compound containing Mg2 and O2 ions whereas Mg s and O2 g are elements with no charges.
The reaction is2 Mg O2 2 MgO. The following questions ask you to provide some details of the chemical processes involved in a reaction between magnesium and oxygen. When this reaction occurs strong ionic bonds form between two oppositely charged particles ions.
From this perspective the reaction between magnesium and oxygen is written as follows. Iron can be transformed to steel an alloy which is more resistant to rust. The term reduction comes from the Latin stem meaning to lead back Anything that that leads back to.
Magnesium has a very energetic combustion reaction with oxygen where two atoms of magnesium bond with one molecule of oxygen gas to form two molecules of magnesium oxide. Chemistry questions and answers. Collect the oxygen in seconds Rate of reaction in cm.
The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium. Magnesium burns so bright because the reaction releases a lot of heat. Table Mass of What is the percent yield of MgO in this reaction.
A student investigated the reaction between magnesium and hydrochloric acid. Magnesium reacts with oxygen to make a compound called magnesium oxide. When magnesium is placed in a flame from a small blow torch it burns quite brightly forming magnesium oxide a salt that contains one magnesium for every oxygen.
The term reduction comes from the Latin stem meaning to lead back Anything that that leads. Oxygen and magnesium combine in a chemical reaction to form this compound. The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium.
An energy diagram for the magnesium-oxygen reaction is shown to the right. Then balance the chemical equation and identify the type of reaction. Magnesium gives up two electrons to oxygen atoms to form this powdery product.
During cellular respiration glucose and oxygen are changed into energy and carbon.

Dat Bootcamp Oc Test 1 5 Flashcards Quizlet

Question Video Calculating Oxidation State Change For Magnesium During Magnesium Combustion Nagwa

Loeblein S Chemistry Phet Clicker Questions Ppt Download

The Enthalpy Of A Reaction Is 1207 Kj Mol Which Of The Following Best Describes This Reaction Brainly Com

Chemistry Nomenclature Chemical Formulas And Reactions Released Test Questions Ppt Download

Which Of The Following Best Describes The Bonding Found Within Al2o3 Brainly Com

Which Best Describes This Reaction Between Magnesium And Oxygen Decomposition And Redox Reactions Brainly Com

Chapter 6 Chemical Reactions Classification And Mass Relationship Ppt Download

Which Best Describes This Reaction Between Magnesium And Oxygen Decomposition And Redox Reactions Brainly Com
Solved Select The Choice That Best Describes The Following Reaction The Course Hero
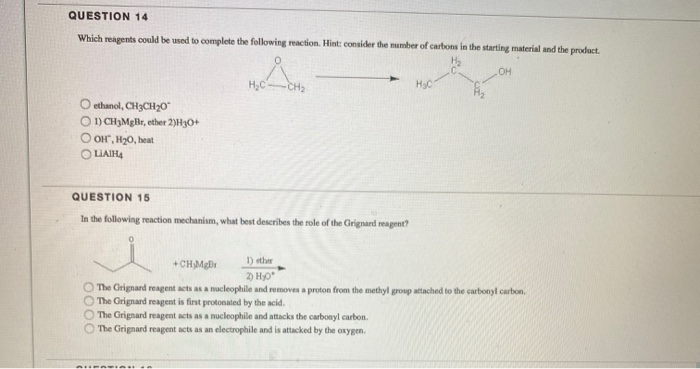
Solved Question 14 Which Reagents Could Be Used To Complete Chegg Com

Chemistry Final Exam Sample Items Which Idea Of John Dalton Is No

Chemistry 2000 Released Test Items Virginia Department Of Education Ppt Download

Chemistry Nomenclature Chemical Formulas And Reactions Released Test Questions Ppt Download

The Law Of Conservation Of Mass Can Be Demonstrated By A Chemical Reaction Which Of The Following Brainly Com
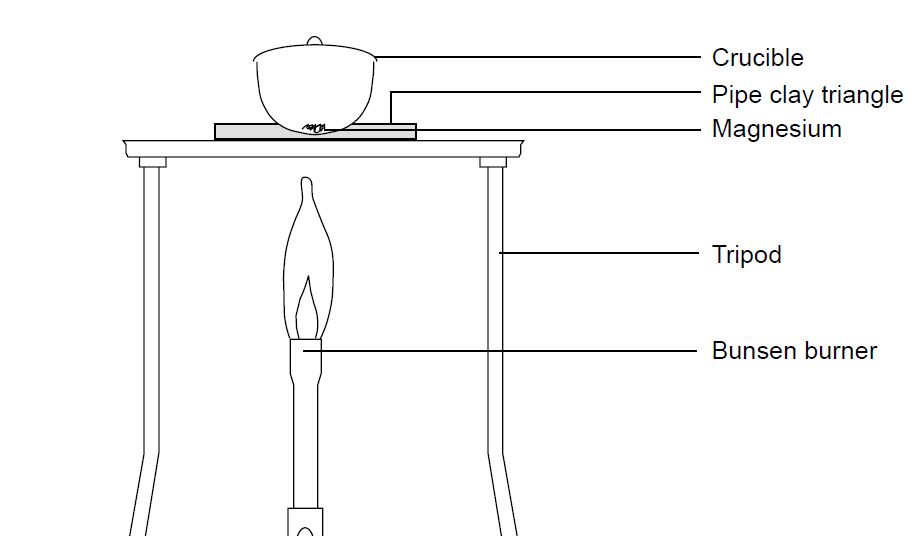
The Change In Mass When Magnesium Burns Experiment Rsc Education

Students Will Need A Calculator And Periodic Table Of Elements Ppt Download
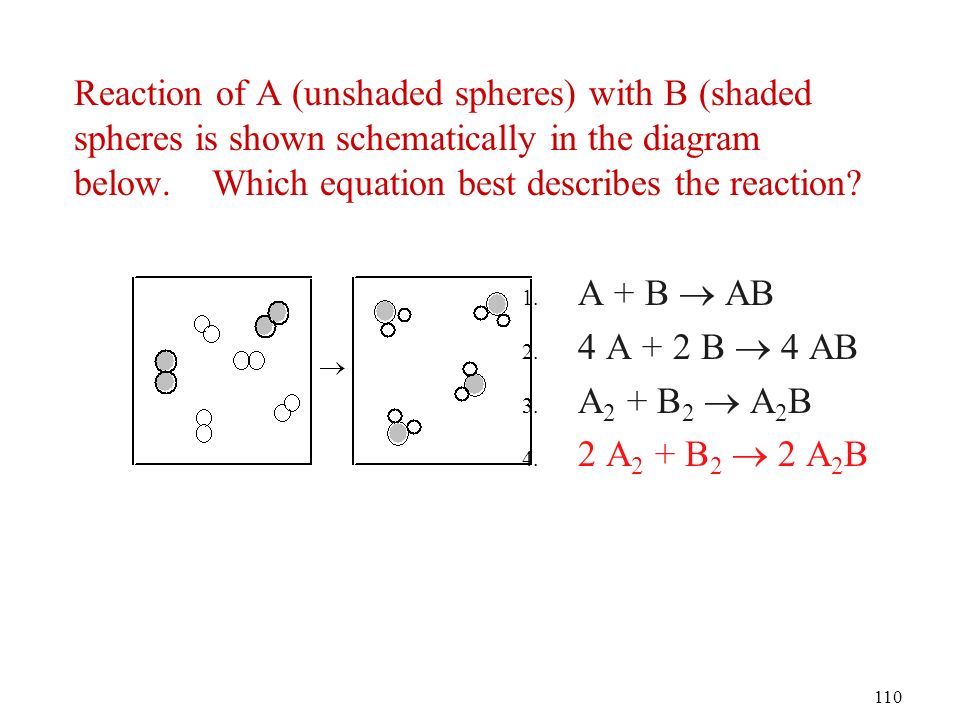
Chapter 6 Chemical Reactions Classification And Mass Relationship Ppt Download
Comments
Post a Comment